
PeterH
-
Posts
1,588 -
Joined
-
Last visited
Content Type
Profiles
Events
Forums
Blogs
Gallery
Posts posted by PeterH
-
-
On 9/5/2024 at 9:46 AM, davelea said:
I've had an initial response back from Paragon saying that it could be the watts had to change for my area/electrical supply. I'm in the UK and the kiln is 11000 Watts 230 Volts, but it says on the same label the max temp is 1288.
Well it was probably worth a try. To state the obvious, talk to Paragon again and emphasize the figures on the kiln plate.
Could you post all the figures on the kiln plate please, and do you have any idea of the resistance of your elements?
> I'm in the UK and the kiln is 11000 Watts 230 Volts,Do you have a special power supply, or are you just running it on the house mains?
PS For the experts.
I have reservations about the use of European “harmonised voltage limits” when applied to heating equipment.
https://leadsdirect.co.uk/knowledge-base/what-is-the-difference-between-uk-voltage-and-european-voltage/Surely the kilns elements should be designed for the "nominal voltage" in the country it is used in (i.e. 240V in this case). I would expect the kiln plate to reflect this stating the nominal voltage and the current at that voltage for the fitted elements.
Running the same elements at nominal voltages of 220V & 240V will generate about 91% and 109% of the power generated at 230V. The differences from 100% being about the same as the point where you consider replacing the elements. (Not to mention the different house-wiring & breaker requirements.)
For the record https://shop.clay-planet.com/paragon-tnf-27-3.aspx and choosing 240V single-phase gives
240V, 48A, 11500W, max 1287C (checking V*A = 240*48 = 11520W) -
I would just
- print it -- either on my computer printer or via a library of office-services print shop.
- then put it into a loose leaf binder, or probably put the sheets into holders and put these into a loose leaf binder.

If you really want it bound into a book I think there are people who will do it for a price. This sort of thing:
https://www.blurb.co.uk/pdf-to-book
https://print2demand.co.uk/from-pdf-to-printed-book/
https://www.prestophoto.com/create/pdf-book-printing
... although they may struggle with the page size the manual comes in.Try googling some thing like :
getting book made from pdf
- Bill Kielb and Pres
-
 2
2
-
After a career in software engineering, is there any chance that it's "finger trouble"? We are all vulnerable, so double-checking can be worthwhile.
For example.
1) Carefully/blindly follow the instructions to display SFTY (maximum temperature) step by step.
2) Look to see what LIM (set maximum temperature) is set to, and then see how high you can set it.
- using the same name for two different things tends to lead to confusion (apparently factory setting and user setting).From a quick glance at
https://www.manualslib.com/manual/1614345/Paragon-Sentry-3-0.html?page=25#manual -
16 hours ago, Callie Beller Diesel said:
Do we know what toxicology info was updated?
As far as I can tell the Mayco SDS have not been updated this year.
My impression is that any changes to Mayco labelling & SDS and formulation were not implemented, due to a variety of issues, including:
After meeting with our supplier, it is their decision to not reformulate to a more user-friendly formula, nor are they willing to replace the antiquated equipment that is causing the packaging problems.An answer to your question might be found by monitoring changes to labeling & SDS of the products of competitors staying in the market.
alternative options such as Laguna Lusters and Colorobbia Lustres are available.
... both for changing risk levels and the disappearance of H&S warning for any "risky" chemicals removed by new formulations.A first step would be to archive the existing SDS for these products. Both from the manufacturers and -- if those are recent -- from pottery shops who haven't updated their copies recently.
- Callie Beller Diesel and Min
-
 2
2
-
6 hours ago, Mark C. said:
If you need a true semi non fluxing cone 10 clear I can post mine again if needed.-The trick is thin application.Firing hot helps as well to keep it clear.I did pot it herte long ago -its called HT 51
I found two previous postings of HT 51, embedded in related discussions.

-
I've not had many dealings with CTM, but their website has always looked competent. So I sent them an email
Hi,
Having a confusing discussion on the web at the moment over the solubility of bismuth subnitrate.
Lots of sources on the web say that it's highly soluble, while lots of other sources say that it's insoluble.
One possible explanation is give in
https://en.wikipedia.org/wiki/Bismuth_oxynitrate (apparently yet another synonym).
Bismuth oxynitrate is commercially available as Bi5O(OH)9(NO3)4 (CAS number: 1304-85-4 ) or as BiONO3·H2O (CAS Number: 13595-83-0 ).Do you know the CAS number of the bismuth subnitrate you sell?
Regards, Peter
Their reply (within minutes) was
Soluble in acid, insoluble in water like most of the heavy metals,
Kind regards
Graeme
CTM Potters Supplies - Doncaster Branch
Simba Materials Limited
Unit 7/8 Broomhouse Lane Ind Estate
Edlington
Doncaster
DN12 1EQ
Tel: 01709 770801 Fax: 01709 770803PS I'll remind you when I was making a "lustre" decades ago (water, bismuth, iron, probably CMC) I think added glycerine to aid solubility/suspension. If it was aiding solubility it might be worth trying with the wet resinate process. If it was aiding suspension it probably wouldn't help.
-
On 8/30/2024 at 7:39 AM, Imu said:
Just a general overview that working with lustres uses the water soluble forms of the metals.
Both the wet and dry processes use metal salts. But surely only the wet process requires the salt to be water soluble. Not least because the dry process doesn't add water and is carried out above the boiling point of water.
Daly, Hainbach and Parmalee all recommend the dry process for bismuth resinate lustre. Probably worth trying it before worrying about the ambiguities of "bismuth subnitrate" solubility.
-
-
31 minutes ago, Imu said:
Bismuth subnitrate is throwing me off a little, as far as I can fathom this is a generic name for a group of bismuth nitrates but I'm assuming it should be a water soluble form which would be H9Bi5N4O22 but cant seem to find much information on buying or making this. I did buy some from a pottery supplier but it doesn't seem as water soluble as I thought, so possibly Bi5O(OH)9(NO3)4, did you use a specialist supplier for bismuth? Was it the water soluble form?
1) H9Bi5N4O22 looks like it's just a short form for Bi5O(OH)9(NO3)4 . Certainly it has the same numbers and types of atom.
2) All the references I'm finding claim that bismuth subnitrate is highly soluble in water. Are you certain that you didn't get bismuth nitrate? Can you share the name of your pottery supplier?
3) I have a lingering memory of having problems dissolving a bismuth compound several years ago. Perhaps related to:
https://www.atamanchemicals.com/bismuth-subnitrate_u25219/
bismuth subnitrate slowly hydrolyses in water
... and even vaguer memories of adding "something" to reduce the effectaha! https://patents.google.com/patent/US2142957A/en
To a water solution of the sodium salt of the mono-substituted carbalkoxy acetic acid is added a molecular equivalent of the bismuth sub-nitrate, in a water solution containing in sufiicient quantity any polyhydroxy alcohol which aids in holding said bismuth sub-nitrate in solution in the water; for which purpose we have found that either glycerol or mannitol is very suitable. A reaction occurs immediately, and without the necessity for heating; and by that reaction the desired basic bismuth salt of the carbalkoxy acetic acid of Formula 1 is formed. If the monosubstituted malonic ester used is the ethyl ester, which we prefer, R is the ethoxy group.
... for the terminologically confused, like myself: glycerol is also called glycerine or glycerin (IIRC the trade may use different names for different purities)4) BTW CTM list bismuth subnitrate
https://www.ctmpotterssupplies.co.uk/rawmaterialstwo.html -
I think that this image helped me understand things a bit better.
From: https://uk.rs-online.com/web/content/discovery/ideas-and-advice/thermocouples-guide
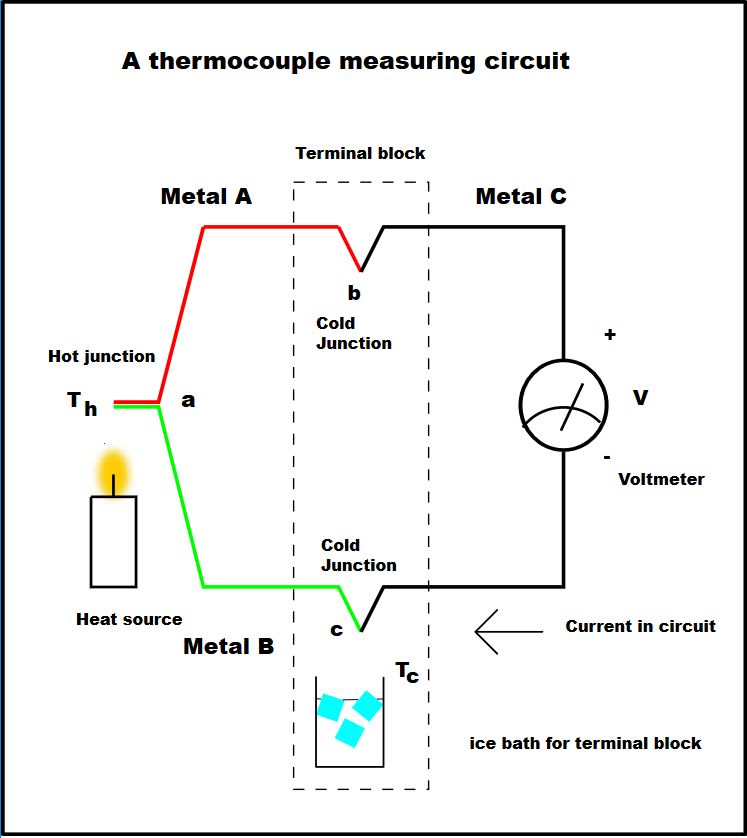 From:
From:
-
On 7/25/2024 at 2:47 PM, Picassowhat said:
Another reason we are leaning toward the smaller (2318D) rather than the larger (2322D) kiln is the lower amperage (35 vs 48). Even if we have the electrical room / capacity for the latter (as our electrician has indicated), we would prefer to place less stress on our electrical system unless there is a very good reason to purchase the larger kiln.
I haven't noticed this mentioned so far ...
What size breaker is your electrician intending to use for those kilns? (It may be worth asking as I understand that some "domestic" electricians are not up to speed on the regulations for equipment like kilns, and it shouldn't be 35 & 48.)https://coneartkilnsshop.com/index.php/product/2318d/
https://coneartkilnsshop.com/index.php/product/2322d/ -
3 hours ago, Retxy said:
searches on the website for part numbers dont yield anything.
Might be of marginal interest re the availability of the 4300 & 4600 timers.
...Which includes
Yeah, I consulted with Paragon before posting here and their kiln tech told me the same thing... Well she said they do have the timers for the ones with two timers (like mine), but apparently some of these Duncan kilns had a single, combined timer that they don't have available anymore. Just saying this to spread the knowledge... -
To me this version of the diagram looks slightly less unreadable
-
>Obviously a very specific technical question here I'm fumbling with. It's for a very large pendant lamp that needs >to be translucent.
>Tried firing porcelain with chuck inside and out. Severe warpage.
>Tried molochite -- still too thin to fire without warpage, also translucency is compromised.Pictures?
All porcelains are not equal, a couple of "background" references.
Warping
https://digitalfire.com/trouble/warping
... including suggestions to address

-
TAGINE POT: A BASIC HOW-TO GUIDE FOR BEGINNERS
https://www.berlingerhausus.com/blogs/new-blog/tagine-pot-a-basic-how-to-guide-for-beginners-1If you a thinking of making one -- making flame-ware pottery is demanding, and it probably still needs to be used in a way that minimizes thermal shock.
Hence the emphasis on insurance in this thread.
-
2 minutes ago, Catatonic said:
and what does "galling" mean
It's a new one to me as well ...
https://en.wikipedia.org/wiki/Galling- Bill Kielb, Hulk and Catatonic
-
 3
3
-
23 hours ago, Jeff Longtin said:
Different from glass setters you will need to make a setter from scratch and fire it with your porcelain form so it shrinks at the same rate.
Making a "bespoke" setter for a cast shape.
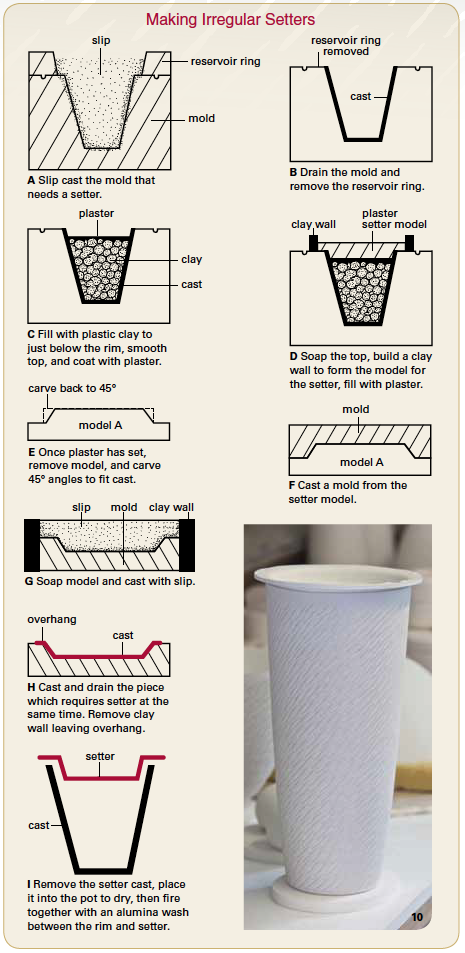 A picture of you "lampshade" and its firing supports
A picture of you "lampshade" and its firing supports
From https://tinyurl.com/mryx92z7A picture of you "lampshade" and its firing supports would help.
-
Two pretty obvious points.
1) Bone china warps more than porcelain (hence the high-bisque low glost). So it's not an answer to warping problems. Can be nice stuff though.2) You may just have to support your form better. Which might also involve a switch to high-bisque low glost.
3) Are you using a setter? Might one help?

A picture of you "lampshade" and its firing supports might help the experts.
PS A thread on somebody trying to fire an inherently unstable form.
-
52 minutes ago, Mark C. said:
There was woman for years here that does colored claty workshops-Her Name is Chris Campbell if I recall -look up her posts on this subject say 6-10 years ago.
Is there anything specific you're thinking of, we've already referenced her colored clay web page.
http://www.ccpottery.com/colored-clay-lessons--chris/how-to-color-clay-with.html@Grace W The search function on this site only examines "active" threads. To search the whole site for contributions by Chris Campbell ...
for google
site:community.ceramicartsdaily.org Chris Campbell
for other search engines
community.ceramicartsdaily.org Chris CampbellPS Note that Chris just happened to register here as Chris Campbell, most people use shortened forms or pseudonyms.
-
8 hours ago, Louisemarion47 said:
My dilemma is that I love the unglazed reduced stoneware And I want my slip drawings to “pop” and contrast with the surface of the reduced stoneware . Perhaps I can paint the clear glaze on the slip drawings.
A half-baked thought.
1) Would a self-glazing slip simplify things? In that it would leave the stoneware unglazed, and the slip would not need to be glazed (which sounds tricky to do accurately).
2) Would something like this work?
https://ceramicartsnetwork.org/ceramic-recipes/recipe/Self-Glazing-Slip-183263#
... although that's for cone 6.
3) Or perhaps a parian slip?
https://www.potterycrafts.co.uk/Products/stoneware-porcelain-clay/PW101
4) Or could you do something similar with efflorescence: e.g. "soda-wash" in the slip. Although you might get a glazed halo round the slip.5) And would things still work in reduction?
-
11 hours ago, Grace W said:
Helpful! I can only find information on adding it to slips and glazes really, which is why I am asking here.
A useful search term is
body stain
... which tries to restrict the search to stains that are specifically recommended for use in clay bodiesYou can tighten the search by adding the name of a major stain manufacturer, such as
body stain MasonIf you want to restrict the search to this site try
community.ceramicartsdaily.org body stainBetter yet, if you are using google try
site:community.ceramicartsdaily.org body stain
... which is how I found a thread that mentioned Chris Campbell's work. Which I then searched for with
Chris Campbell body stainBe aware that some stains have unfortunate colour reactions with some glazes (and possibly some bodies).
PS Bold used for emphasis only.
- Grace W, Callie Beller Diesel and Hulk
-
 3
3
-
2 hours ago, Grace W said:
Helpful! I can only find information on adding it to slips and glazes really, which is why I am asking here.
You might start with:
HOW TO COLOR CLAY WITH MASON STAINS
http://www.ccpottery.com/colored-clay-lessons--chris/how-to-color-clay-with.html

-
58 minutes ago, Mark C. said:
There is a new mold book out now thru ceramic arts network on mold makeing by Johnathan Kaplan. Its called Mold -Making manual
Flier and sample pages at
https://mycan.ceramicartsnetwork.org/s/product-details?id=a1BUe0000004qxhMAA- Hulk and KID-IN-CLAY
-
 2
2
-



Sentry 3.0 SFTY setting
in Equipment Use and Repair
Posted · Edited by PeterH
Looking like it's a genuine 230V kiln and I assume your house wiring is the UK nominal 240v supply?
PS
Kiln plate shows 230V 48A 11000W, while the 240V model is 240V 48A 11500W
So they have probably multiplied the element resistance by (230/240) to maintaining the current, which would reduce the wattage to ~11000W.
I cannot see how this would affect the max safe temperature when run at 230V.
On a 240v supply the current would presumably rise from 48A to about (240/230)²*48A = 52A, which must reduce element life and change the wiring and breaker requirements.
Have to agree with Bill. It really looks like he has a sentry controller programmed for a lowfire kiln, however it got there. I suggest asking paragon how can the safety be raised to that stamped on the kiln.
But you probably have 230V elements, which isn't good if you are running it off a 240V supply.