
PeterH
-
Posts
1,493 -
Joined
-
Last visited
Content Type
Profiles
Forums
Gallery
Posts posted by PeterH
-
-
Can anybody explain the apparently white areas around the red line and dots

-
22 minutes ago, Suresh Sundaram said:
I’m trying to create the look that’s on the cover of Robin Hopper’s Making Marks book

-
> I was looking for the trial version of the NIST? phase diagram software ...
Just to point out that there is a time-dimension to phase transforms that phase-diagrams don't capture.
A physicist might say that they are "thermodynamically correct", and only show what phase has the lowest energy under the given conditions. Saying nothing about how long the change of phase will take.
AFAIK it's rarely of practical significance, although I suppose it's relevant when the timing/temperature of the firing becomes important: e.g slow-cooling microcrystalline glazes, nursing macro-crystalline glazes.

The effect is spectacularly evident in the phase diagram of carbon.

Normal temperature and pressure is about 1 bar & 300K. So every diamond you have seen is about 1,000 bar away from a point where it is "stable" ... and not much closer during the geological time where it was in fairly-near-the-surface rocks.
PS
https://www.whiteflash.com/diamond-education/diamonds-how-do-they-form/

-
6 hours ago, GEP said:
Unity formulas, flux ratios, boron charts are all useful guidelines, but glaze chemistry has far more variables than us mere humans can test. No substitute for first hand experience. “Melt and see” is still an indispensable mindset.
Hard to argue with that, but I would suggest that they can help reduce the number of tests you need to make.
For glazes that phase-separate (e.g. many mattes) the UML formula will not accurately reflect the composition of the different phases. Which raises issues of interpretation and limit setting.
- Kelly in AK and Min
-
 2
2
-
It looks like this URL may show both sides of one of the 3 parts of 183F
-
26 minutes ago, Jeff Longtin said:
If anyone tries mixing this recipe please post your experience. No idea what "petalite" is, or how it acts in a slip body, but I'm certainly curious?
https://digitalfire.com/material/petalite
Petalite is a lithium aluminum silicate mineral (more simply a lithium feldspar) that is commonly used in clay bodies. It is valuable because it provides an insoluble source of lithium and has the highest Li2O:Al2O3 ratio of any natural mineral. Lithium is a strong alkaline flux and is effective over all temperature ranges. It imparts lower expansion and gives a unique color response to copper and cobalt in glazes. Some commercial versions have a chemistry that fairly closely matches the theoretical chemistry given here.
Petalite is most prized for its mineralogical properties. It is especially valuable in imparting thermal shock resistance to clay bodies because it has almost zero expansion when heated above 700C. Bodies with 60%+ petalite can take a direct flame and rapid water cooling without failure. To make a plastic clay body mix petalite with as much ball clay as needed. For a casting body, use as much kaolin as needed to achieve the desired casting properties. Bentonite can be added to either type of body to increase the petalite proportion.
One serious problem with low expansion petalite bodies is that it is very difficult to achieve glaze fit. All common glazes will craze. This is compounded at lower temperatures where the limited low-expansion silica and alumina necessary for melting raises glaze expansion. For some low-expansion bodies, it is almost impossible to match a glaze -
On 1/1/2024 at 10:59 PM, hotzn said:
Is it a problem that the drying clay will shrink onto the inner mold - maybe even leading to cracking?
Yes -- and it will probably be impossible to de-mould.
... although I have a very vague memory that I seen mention of a sacrificial "core" -- which burns-out/crumbles during the firing -- being used in some circumstances.Somewhat tongue in cheek, a picture of ancient Peruvian pan-pipes from
https://digitalassets.lib.berkeley.edu/anthpubs/ucb/text/nap002-003.pdf

My understanding of the paper is that the pipes were cast individually and then joined by adding additional clay by hand (although the set of individual pipe moulds for a complete panpipe were held in a common mould structure).Seriously, what is the intended usage of your end-product. It will enable us to understand the importance of the precision you seem to be demanding. It might also mean that the inevitable shrinkage & warpage of clay products during both casting and firing may render your product unusable for your intended purpose (a point already raised by Mark).
-
Any idea of the manufacturers code for this item?
For example looking through
https://img1.wsimg.com/blobby/go/a473195b-32be-4831-aad8-e122a50bcf46/IN THE KILN CERAMIC GREENWARE-BISQUE LIST.pdf
gives

... but I failed to find more details on the site.Searching for 183F elsewhere give:
https://bisquebusters.com/products/scenery-mountain-train-3pc
A more general search found this, but without a manufacturers code
https://www.ebay.co.uk/itm/134018515130

- Jeff Longtin and Chilly
-
 2
2
-
4 hours ago, Suresh Sundaram said:
I found Nigrosh's casting slip recipe on Glazy. It's listed as a glaze!
There are also a couple of Nigrosh's Egyptian Paste recipes under glazes.
PS Googled site:glazy.org Nigrosh
-
6 hours ago, Jeff Longtin said:
Leon Nigrosh wrote a nice book about "low fire" back in the 70's that I always found helpful.
Low Fire : OTHER Ways to Work in Clay Leon I. Nigrosh?If this is the right book 2nd hand copies seem quite cheap (<$5).
-
Are you trying to make a set of panpipes or a high-tech industrial component? It would help if you gave us an idea what you are trying to make, and the use to which it will be put. This gives the experts some criteria to judge the merits of alternatives production techniques.
Two minor points.
- If you use multi-part moulds don't draft-angles become irrelevant, although parting lines may require tidying.
- Is extrusion an option? -
I was fascinated by mainstream and "alternative" photographic techniques as a child. So some book knowledge and no practical experience with images on clay.
What is the name of the process you are using?
@blackthorn has been a/the major contributor to several threads on photo-lithography and other image transfer techniques.
He seems to have tried many processes, often with mixed success, and the best results I remember are from a ink-jet printer based idea, mentioned in a weakly-related thread.
In reply to your specific questions:
There are several types of linseed oil (raw, refined, stand, etc.) and it seems like they have either recommended a particular type (all different) or just said "linseed oil".
Some say to use gum arabic and others don't mention it.
There are lots of process and gum arabic is used in at least two different ways.
1) Relying on the fact that oil and water don't mix.
For example a very simple process of taking an image from a photo-copy.
Photolithography on Clay
https://pistrucciartworks.wordpress.com/2011/03/20/photolithography-on-clay/2) Using gum as the basis of a photo-emulsion, where the gum hardens when exposed and the unexposed gum can be washed away. [Having the same problems as newsprint -- limited tonal range and sometimes needing the use of half-toning.]
Some process issues
Trouble w/ Cyanotype on BisquewareDiscussion of three variations on the theme: classical cyanotype, gum bichromate and gum with masion stains.
PS There are H&S issues with gum bichromate
-
11 hours ago, Bill Kielb said:
I guess to each their own. I think lots of explanations sound complex, most materials don’t really care though. I find treating clay just like we do in construction, the basic properties of materials, has greatest clarity and predictability of outcome.
Residual stress sounds good but does not have any meaning to me, it does not add any clarity. When I compress am I adding stress? Residual? What is residual?
Memory is too abstract for me as well, one side is stretched, the other compressed. Fold a piece of paper, see why there is a crease there and why it’s difficult to completely remove the crease without altering the local properties of the material to match the surrounding material. Clay has plastic limits like most other material, exceed them and the results are predictable. - to me, memory is a cool thought but again does not really add clarity to what is happening.
Two semesters of hydraulics and hydrology help but definitely not the end all for clay. Still has worked well for me constructing roads, sidewalks earthen dams, sub grades, buildings …. Sneaking up on half century now.
Just my take on this.
Certainly as a term "memory" has its problems, not least the implication of some sort of consciousness.
However "local platelet alignment" is a real property, that can cause the previous history of the clay to influence its future behaviour. An effect that sometimes has practical significance.
Techno File: Clay Memory
https://ceramicartsnetwork.org/ceramics-monthly/ceramics-monthly-article/Techno-File-Clay-Memory#Crack H in Hamer & Hamer
http://ceramicsfieldguide.org/pdf/materials-handouts/ClayCracks.pdf

PS
"Squeezing out water" may sometimes relate to changing platelet alignment so there is less inter-platelet space to hold residual water.
A similar effect occurs during hard-panning, but in this case caused by excessive deflocculation rather than physical manipulation.

-
31 minutes ago, Judith B. said:
if anyone knows or has an idea of what the ingredient is that gives Tangerine Ice and Ruby Dust (among other glazes in that group) that dappled lighter coloring?
Tangerine ice Ruby Dust


-
I've no practical knowledge, so treat this all a here-say
I'm interested in how you get on, but ...
- I don't think either bones -- or seashells -- will survive high firing sufficiently intact to act as a stilt.
- I doubt that bones will help form a "glaze" in the same way that seashells do.Firstly I think shells are used as a separator between the pot and clay wadding placed inside the shell. Shells seem a much better shape for this than most bones.
Effective Side Firing
https://ceramicartsnetwork.org/pottery-making-illustrated/pottery-making-illustrated-article/Effective-Side-Firing#
While wadding is traditionally only used in atmospheric kilns to prevent pots from sticking to the shelves, it is necessary for side firing in electric kilns. Sea shells turn to powder (calcium oxide) during the firing, and will not support the weight of a pot. Using wads under the shells prevents the pot from falling onto the drip tray and sticking to it.Secondly the chemistry is different, perhaps critically so.
Shells in Ceramics
https://www.weloveclay.com/read/53007/53007/
Why then were shells not adopted more widely? Australian wood fire potter Owen Rye has pointed out that though ancient potters were keen observers of their craft, none could have understood or intuited the science of shell use. The shells themselves are formed from calcium carbonate which, when heated, converts to calcium oxide or ‘quick lime’, a powerful flux. In isolation, calcium is one of the most refractory oxides, only melting at 2572˚C+. But combine that calcium with alumina and silica and you have what is known as a ‘eutectic’ – a combination of oxides that melt at a lower temperature than they would on their own (in this case, a much more manageable 1170˚C).Why then were shells not adopted more widely? Australian wood fire potter Owen Rye has pointed out that though ancient potters were keen observers of their craft, none could have understood or intuited the science of shell use. The shells themselves are formed from calcium carbonate which, when heated, converts to calcium oxide or ‘quick lime’, a powerful flux. In isolation, calcium is one of the most refractory oxides, only melting at 2572˚C+. But combine that calcium with alumina and silica and you have what is known as a ‘eutectic’ – a combination of oxides that melt at a lower temperature than they would on their own (in this case, a much more manageable 1170˚C).
All this means that on the interface between shell and pot there is a melt that leaves a shell-like scar on the surface of the clay. As the shells are stuffed with clay to prevent them from collapsing, the same happens on the inside. The core of the shell remains pure calcium, which is dry, friable, expands when wet and can be easily removed after the firing. An aesthetic bonus of using seashells is that they contain small quantities of salt. During the firing, this salt can volatilise, leaving a subtle halo of salmon pink or orange on lighter coloured clay bodies.Shells decompose into calcium oxide, bone eventually decomposes to a mixture of calcium oxide and phosphorous pentoxide, which may well prevent a suitable eutectic forming. Also bone ash tends to retain some of its structure, probably making both oxides less available for forming a "glaze".
https://en.wikipedia.org/wiki/Bone_ash
Bone ash is a white material produced by the calcination of bones. Typical bone ash consists of about 55.82% calcium oxide, 42.39% phosphorus pentoxide, and 1.79% water.[clarification needed] The exact composition of these compounds varies depending upon the type of bones being used, but generally the formula for bone ash is Ca5(OH)(PO4)3. Bone ash usually has a density around 3.10 g/mL and a melting point of 1670 °C (3038 °F). Most bones retain their cellular structure through calcination. -
-
38 minutes ago, Min said:
it would probably be a good idea to start a new thread on this, would make it easier to find in a search.
+1 for starting another thread.
I'm used to seing rather chunky rubber block moulds on the net. As from 6:53 in this video.
How thick are you printing the walls? Would these rubber moulds be self-supporting when casting into them, or would they need a "backup" mould to prevent distortion?
... which I believe is common practice in some areas.
How to Make Backup Mother Moulds https://www.aldax.com.au/backupmould.htm -
5 hours ago, Bill Kielb said:
Not doubting that especially if the old connections are failing and heating things or the wire is heating the cabinet because its resistance is rising. Gaining. more resistance will not burnout relays it actually decreases the current through the contacts. Just trying to understand how to test for this.
I had a vague memory of possibly related postings using IR thermometers and/or IR cameras. Having been impressed by images such as this one showing an overheated relay lead.

Turns out it was from one of your postings, What's your current position on these techniques?
PS I also have a vague feeling that some mobiles were sufficiently sensitive to IR to give useful images.
-
Help, I'm having trouble understanding these figures.
8 hours ago, Lilith Rockett said:The amps are 28-23-28.
Problem 1.
28+23+28=79Aamps, but the 240V kiln seems to be rated at 60amps.
https://www.sheffield-pottery.com/SKUTT-KM1231-3PK-240V-1PH-p/skkm12313pk.htm
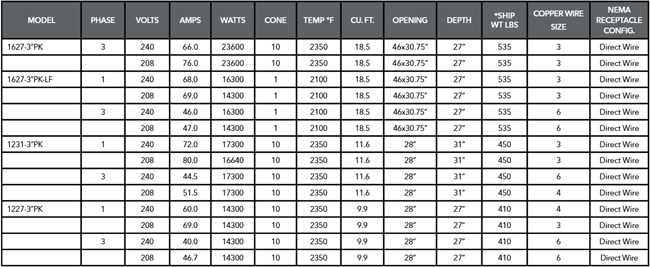
... note that 79/sqrt(2)=55.9, so it might be interesting to know if the current sensor is reading RMS or not.Problem 2
Currents of 28+23+28 indicate that the centre section has a higher resistance than the end sections.But the ends seem to have 2x 8.9ohm elements in parallel, and the centre 3x 11.3 ohm elements in parallel.
https://skutt.com/images/KM1231PK-1PH-and-3PH.pdf
https://www.armadilloclay.com/uploads/5/1/2/8/51288343/element_resistence.pdfSo, ends should be 8.9/2=4.45ohms & the centre=11.3/3=3.76...ohms.
... where the centre has a lower resistance than the ends
... and the currents are very high at 240*2/8.9=53.9amps, 240*3/11.3=63.7amps and 240*2/8.9=53.9amps compared to 28-23-28. -
You may find these of interest if you are going to make your own paperclay.
HOW TO MAKE PAPERCLAY | Chris Campbell
http://www.ccpottery.com/colored-clay-lessons--chris/how-to-make-paperclay-.html
Great if you have a heavy-duty mixed. If you don't you may have to resort to adding the damp paper-pulp to a pre-made thick clay slip, or adding dry clay powder to a wetter paper-pulp mix. In either case it will take longer to dry on a plaster slab due the the greater water content.Making Paper Clay Storage Easier and Less Stinky
https://ceramicartsnetwork.org/daily/article/Making-Paper-Clay-Storage-Easier-and-Less-Stinky -
Interesting to be reminded of prior work, but from pp74-75 of the 2nd edition of Contemporary Studio Porcelain by Peter Lane (2003).
The methods used to make the components for constructing his sculptural installations were developed during a ten-year period of intensive experimentation and intensive research.
...
The procedure of actually casting thin slabs of porcelain is the the most difficult part of the whole process of manufacture because small mistakes can create big problems. The most common faults resulting from incorrect casting are: cracking, warping, and distortion with corners curling to destroy the flatness.The details of the process cover about a page of A4.
Rolling paperclay seems likely to be a lot easier and more fault-tolerant.
PS If you are interested in the book, it's available from A$40 via
https://www.bookfinder.com/search/?full=on&ac=sl&st=sl&ref=bf_s2_a1_t1_1&qi=iA,O6ra27IBsie2OptRYjTAhne4_1702997710_1:9810:16802
... check against other sources such as Amazon -
3 hours ago, Lilith Rockett said:
Perry absolutely does not think it could be the elements, but if everything else has been changed, what else could it be?
I would take a very careful look at Neil's suggestion.
On 12/15/2023 at 8:40 PM, neilestrick said:This is a long shot, but 3 times in the last 20 years I had kilns similar in size to yours have a very strange stalling problem. It turned out to be an electrical interference issue (there are a lot of magnetic fields and whatnot created by the elements and bricks), and the solution was to make sure the controller was directly grounded, not just through the transformer. It's an easy fix and worth trying if yours isn't already grounded that way. Take a look at the backside of your controller, there needs to be a wire that goes directly from the Center Tap terminal to the grounding stud. Not to the transformer or anywhere else first. You may need to get a terminal doubler so you can add another wire.
A very plausible cause of baffling problems, and you can check if it's causing your problems with a very simple wiring change.
As Neil says it's a long shot, but if electrical interference is the cause it's going to be practically impossible to identify by normal diagnostic procedures.
To me trying his suggested fix is a no-brainer, the cost/potential-benefit is so one-sided.
... but I'd continue exploring other options in case it doesn't work out.
-
22 hours ago, Erika gof said:
I am wanting to make VERY thin sheets of clay so they are like paper.
I'm certain you will enjoy experimenting with paperclay.
Just measured my printer paper, it's 0.06mm thick (a stack of 500 sheets measures 3cm). I cannot see you getting anywhere near that.
OTOH playing with home-made paperclay years ago I achieved 2-3m very easily with a rolling pin. I just rolled it out on under a cloth on a slightly porous surface and draped it onto a balloon. Handling thin sheets, especially when drying, may be an issue.
Pouring slip seems to be trying to cast it. I cannot see you getting thin sheets this way -- or getting them off the casting surface either.
Weakly related article.
THE MAKING OF PAPERCLAY PORCELAIN BANNERS
https://www.grahamhay.com.au/harrison1998.html
I mention it because he achieves 1.0-1.5mm with rolling. Which I suspect you could do with most paperclays.
Using a light weight cellulose/cement batt as a backing, spread a jumbo garbage bag over the batt, held in place with paper clips. Spead a layer of paper clay mix fairly evenly over the plastic sheet with a spatula. Place another sheet of plastic garbage bag over the top and roll out the clay in all directions with as much pressure as you can muster until it oozes out off the batt on all sides. Alternatively, the batt can be placed on the slab roller and reduced in that way. Keep rolling until it is as thin as you can get it.

PS Not relevant to you, but an interesting idea I haven't seen before, using ceramic fibre to strengthen the clay during firing (after the paper has burnt out). He apparently needed to do this because he was using very highly/deeply textured sheets.
Firing is to Orton Cone 8 in 4 to 8 hours, depending on the decoration. If the marks are many and vigorous, a longer firing is required to stop the tile splitting up along the incisions. This is why the ceramic fibre is added, as it doesn't bum out like the paper but persists. The paper does a sterling job at room temperature of binding the surface together but the tell tale waft of smoke at 250,C spells its end, and that is when the normal paperclay tile will crack if the fibre isn't added, as the fibre remains intact until elevated temperatures resisting any tendency in the tile to crack apart along the stress lines created in the decoration. Eventually the ceramic fibres dissolve into the ceramic body glass, which is created by the high proportion of nepheline syenite in the recipe.
-












Phil Rogers Ash Glazes book
in Clay and Glaze Chemistry
Posted
Now lots available at £24 over here
https://tinyurl.com/2p4k2jst